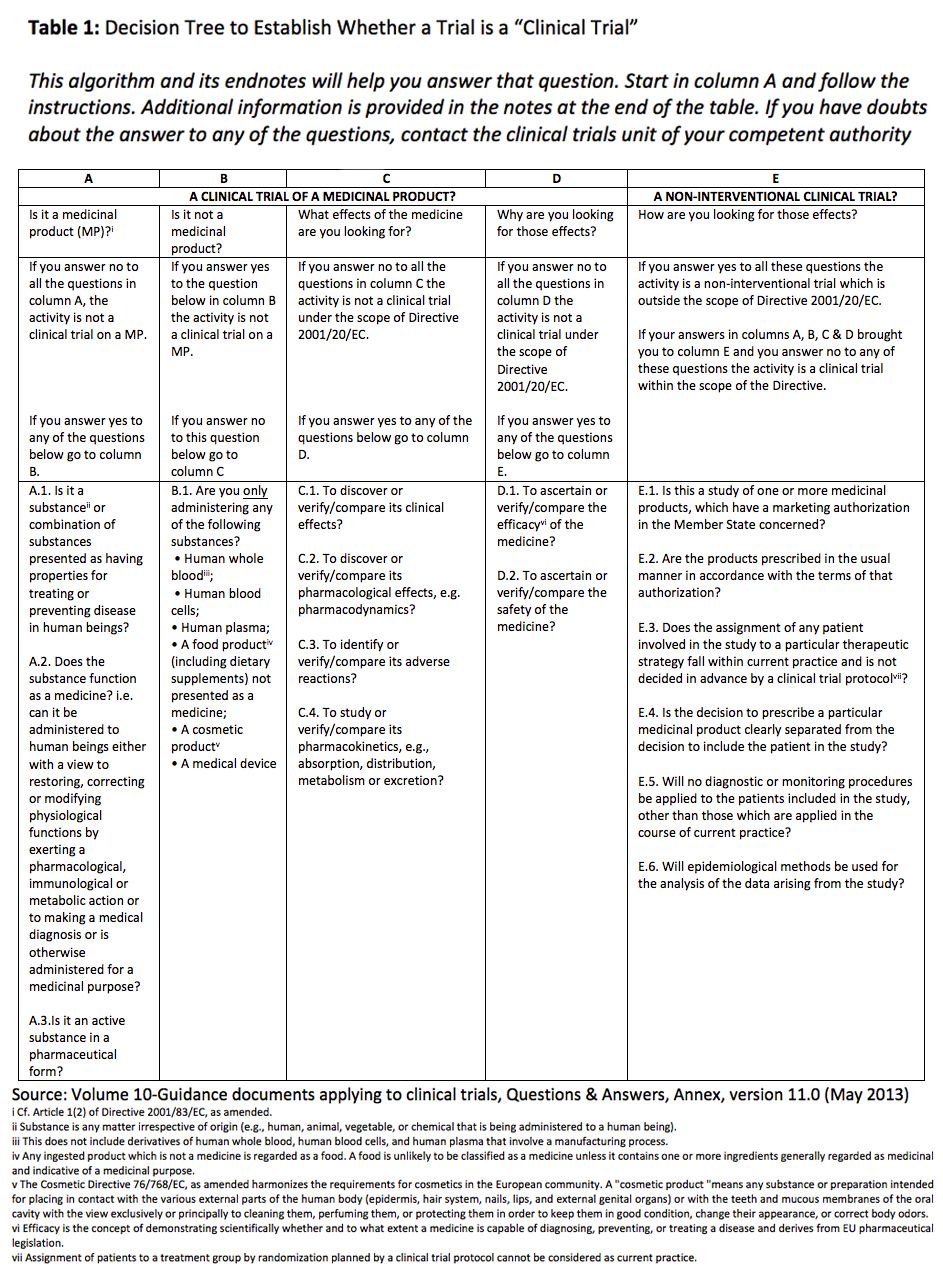
Page 1 of 5 MONITORING SERVICES FOR A CLINICAL TRIAL EXP_17_2019 Background The Barcelona Institute for Global Health, ISGlobal
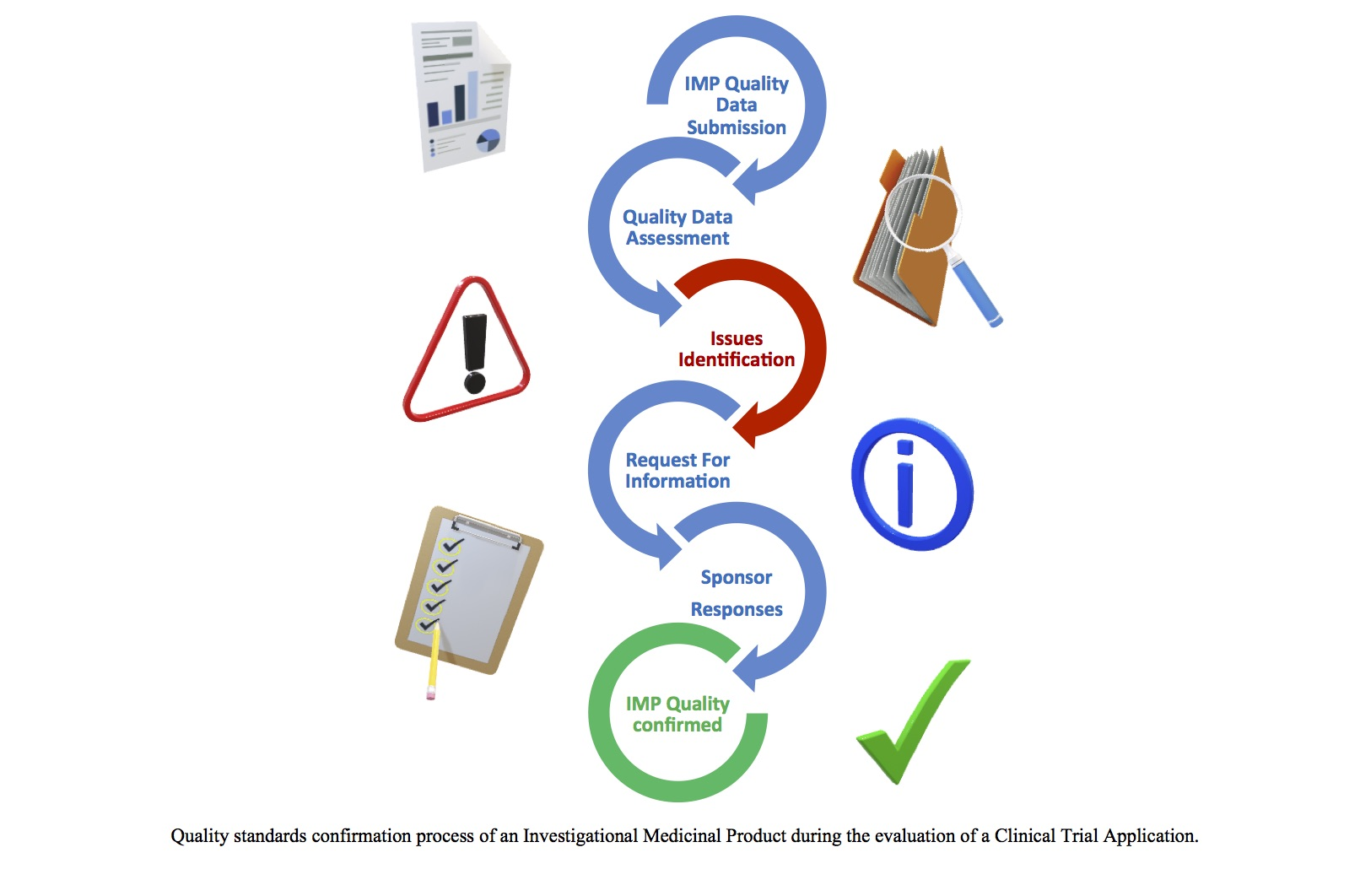
Pharmaceuticals | Free Full-Text | Quality Assessment of Investigational Medicinal Products in COVID-19 Clinical Trials: One Year of Activity at the Clinical Trials Office | HTML

Solve Expiry Labels, DtP, and Timelines for EU 536/2014 Clinical Trials Regulation | Healthcare Packaging
Annex 1: CLINICAL TRIAL APPLICATION FORM (CTA) To be completed by Applicants for all Clinical Trials Study Title: Protocol No:
REQUEST FOR AUTHORISATION TO THE COMPETENT AUTHORITY: REQUEST FOR OPINION OF THE ETHICS COMMITTEE: A. TRIAL IDENTIFICATION
REQUEST FOR AUTHORISATION TO THE COMPETENT AUTHORITY: REQUEST FOR OPINION OF THE ETHICS COMMITTEE: A. TRIAL IDENTIFICATION