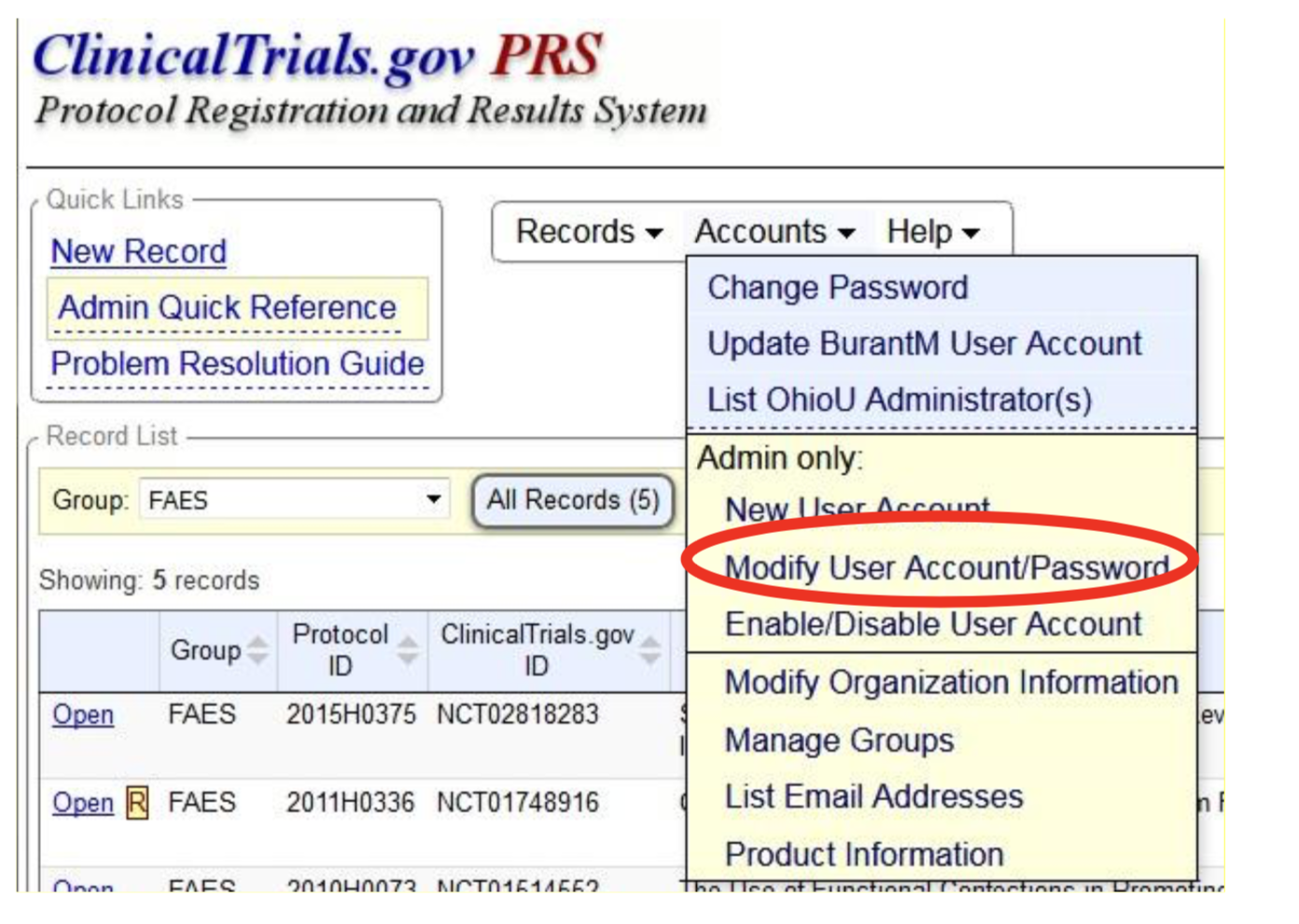
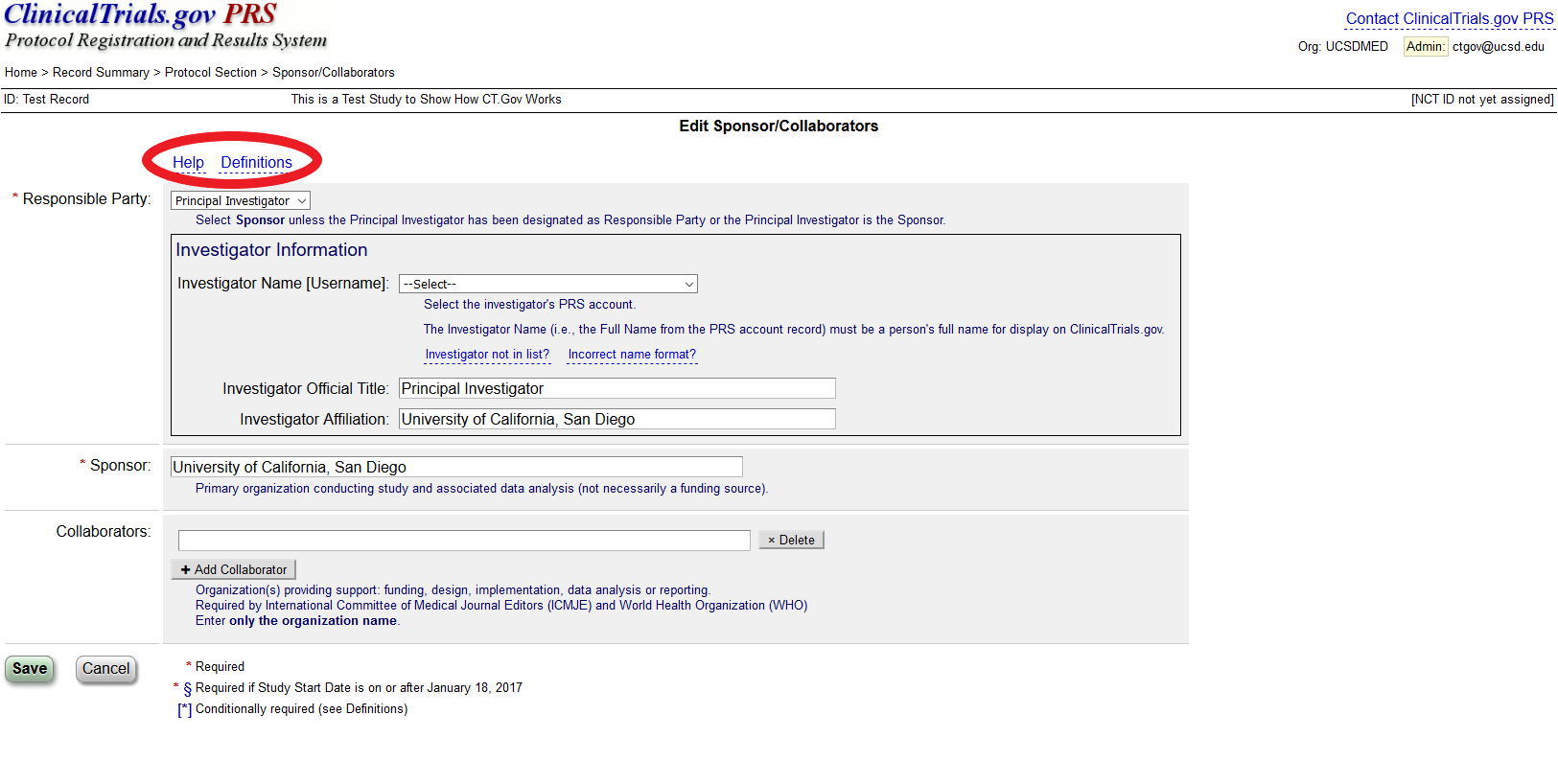
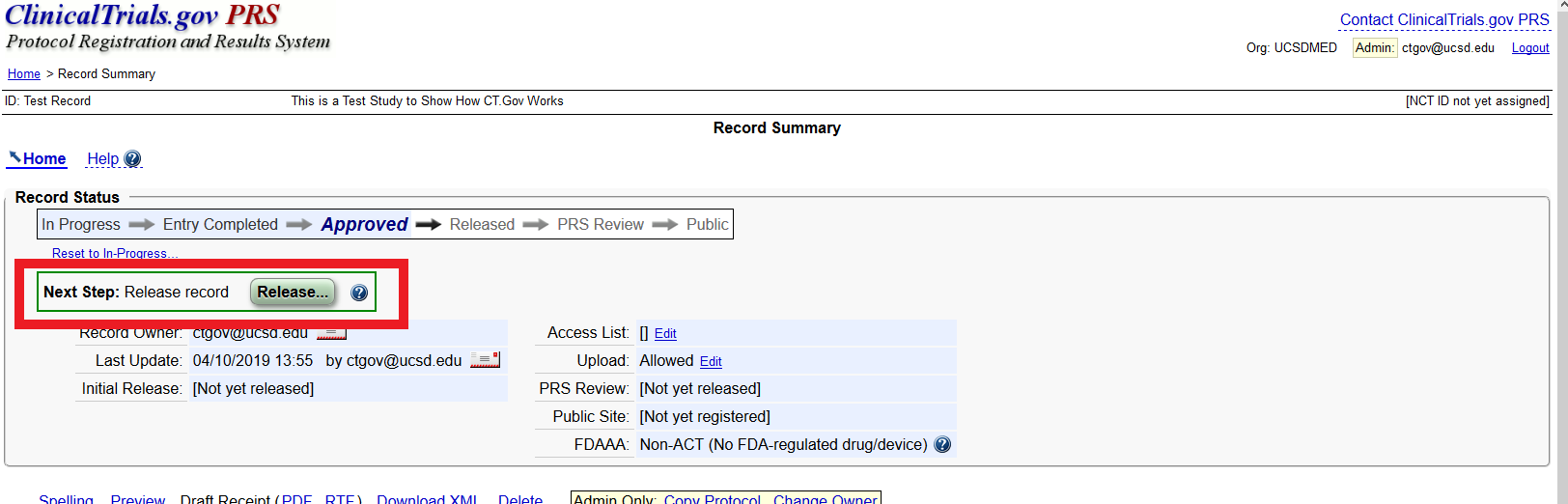
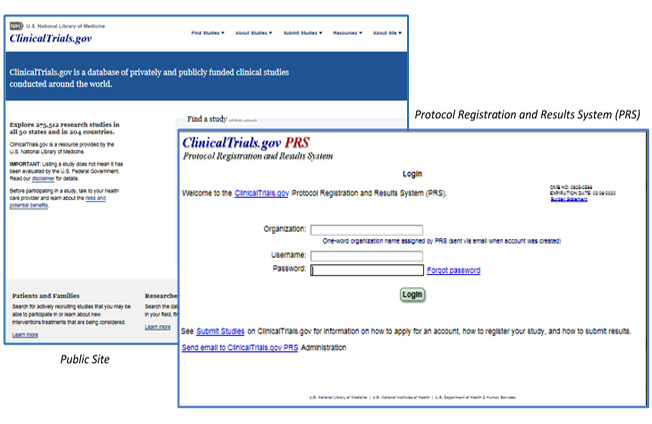
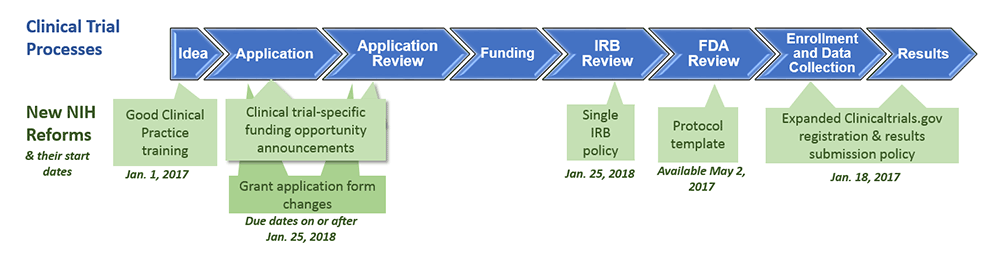
Compliance with legal requirement to report clinical trial results on ClinicalTrials.gov: a cohort study - The Lancet
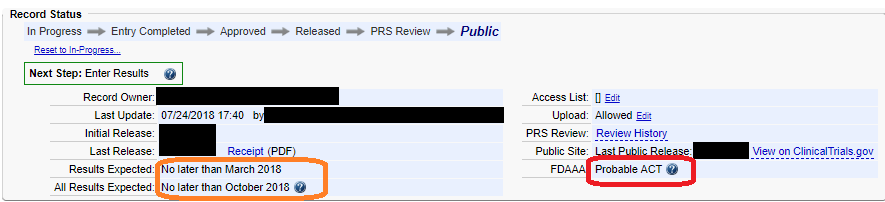
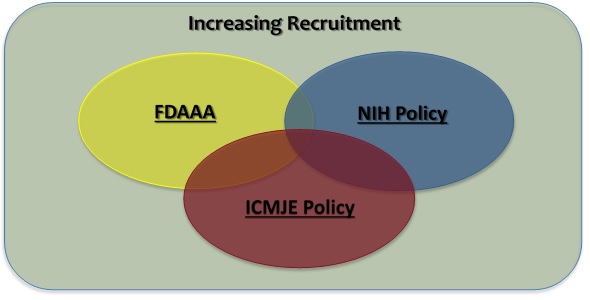
ClinicalTrials.gov Tip of the Week: Seife et al. v. HHS et al.: Results Must be Submitted for “pACTs” to Clinical Trials.gov

ClinicalTrials.gov updates the PRS Guided Tutorials, step-by-step instructions for data providers - NCBI Insights

Medical Writing | Public Disclosure | Clinical trial results disclosure on ClinicalTrials.gov and EudraCT