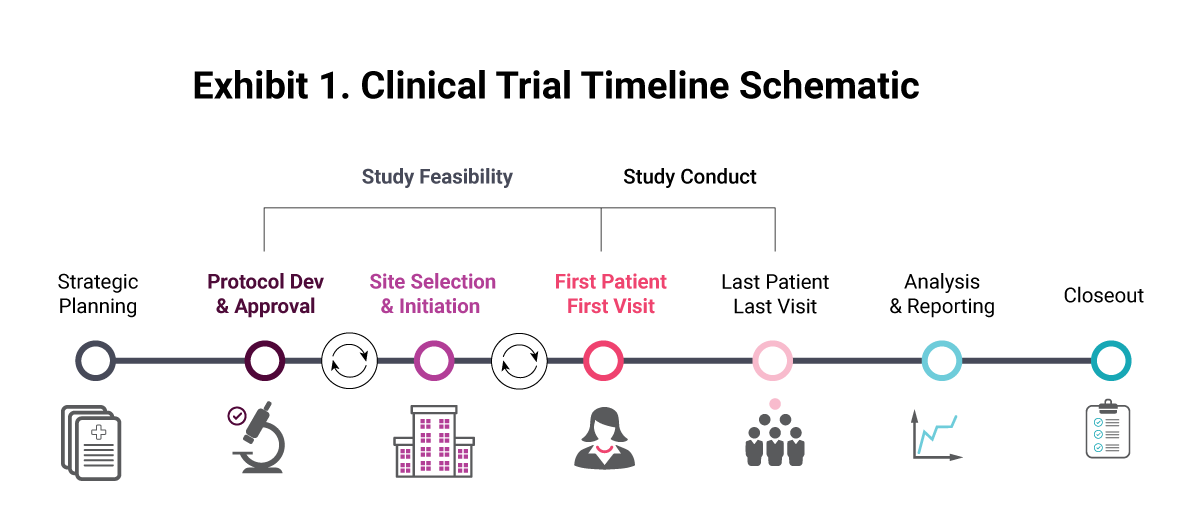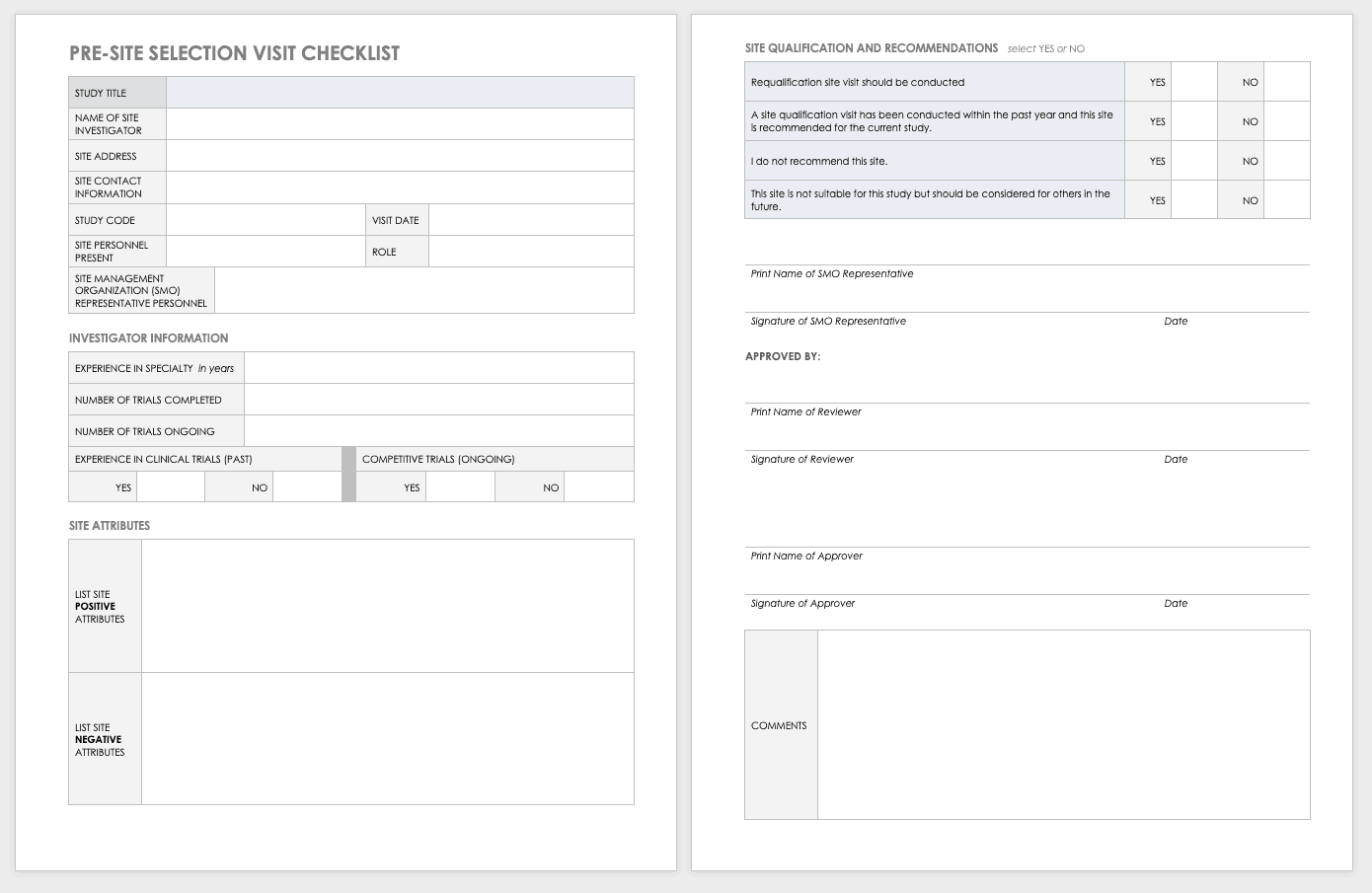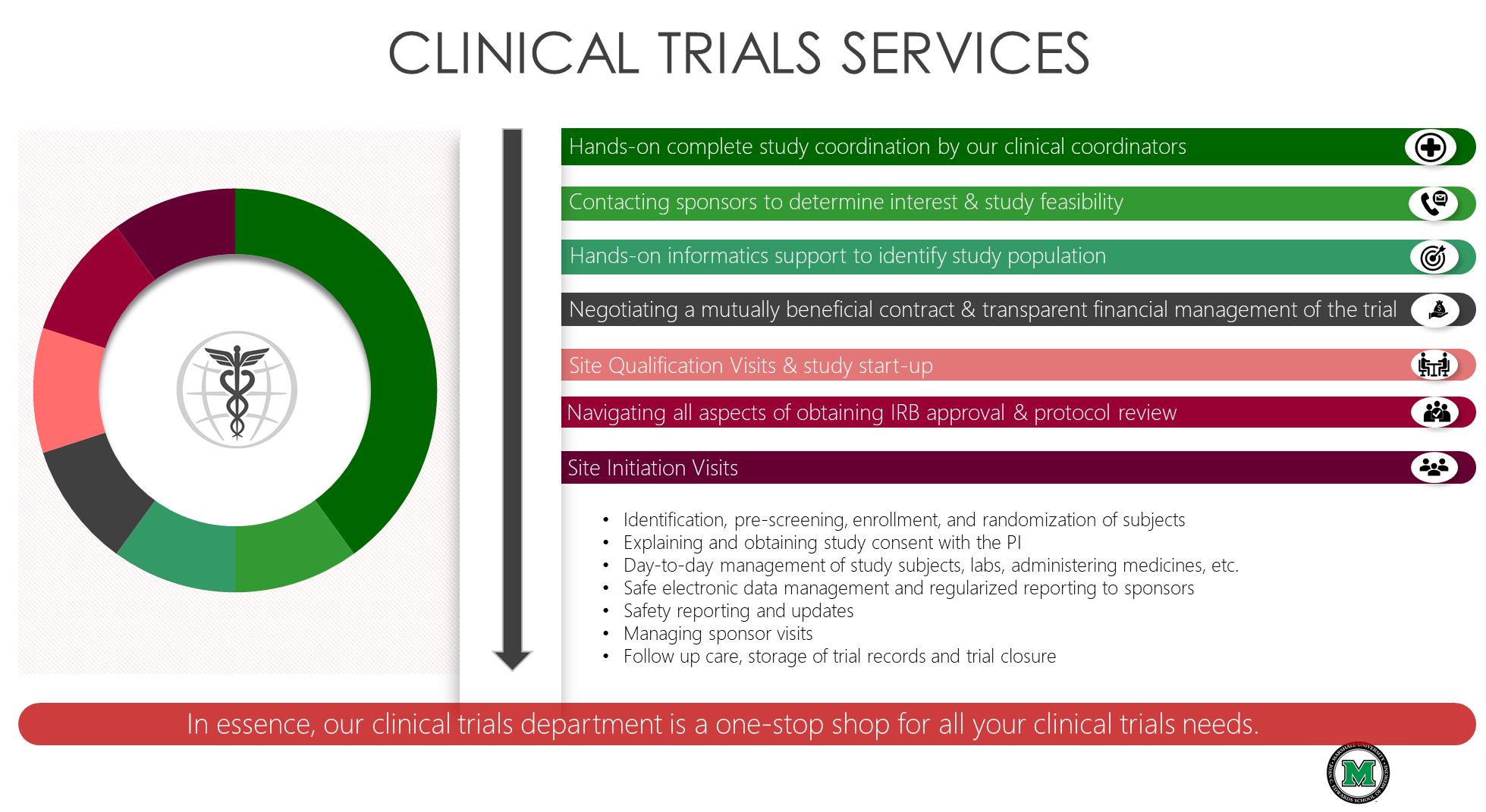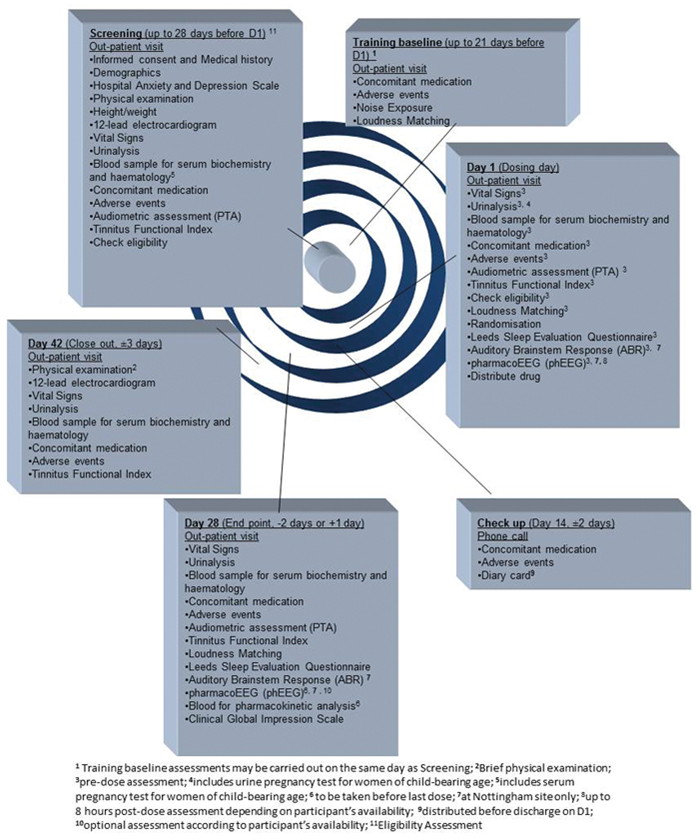
A place for everything and everything in its place: the practicalities of randomised clinical trials | ENT & Audiology News

CT08: Clinical Trial Monitoring: Study Monitoring, Documentation and Closure | Zenosis – Learning for Life

Site Closeout Visit Readiness Checklist v20 1375 | PDF | Clinical Trial | Information Technology Management