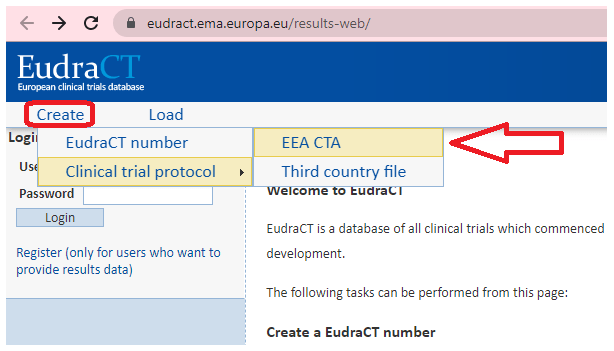
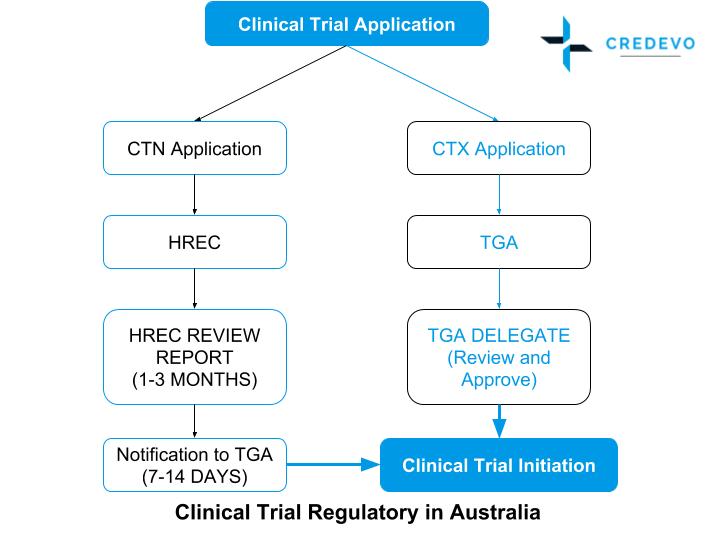
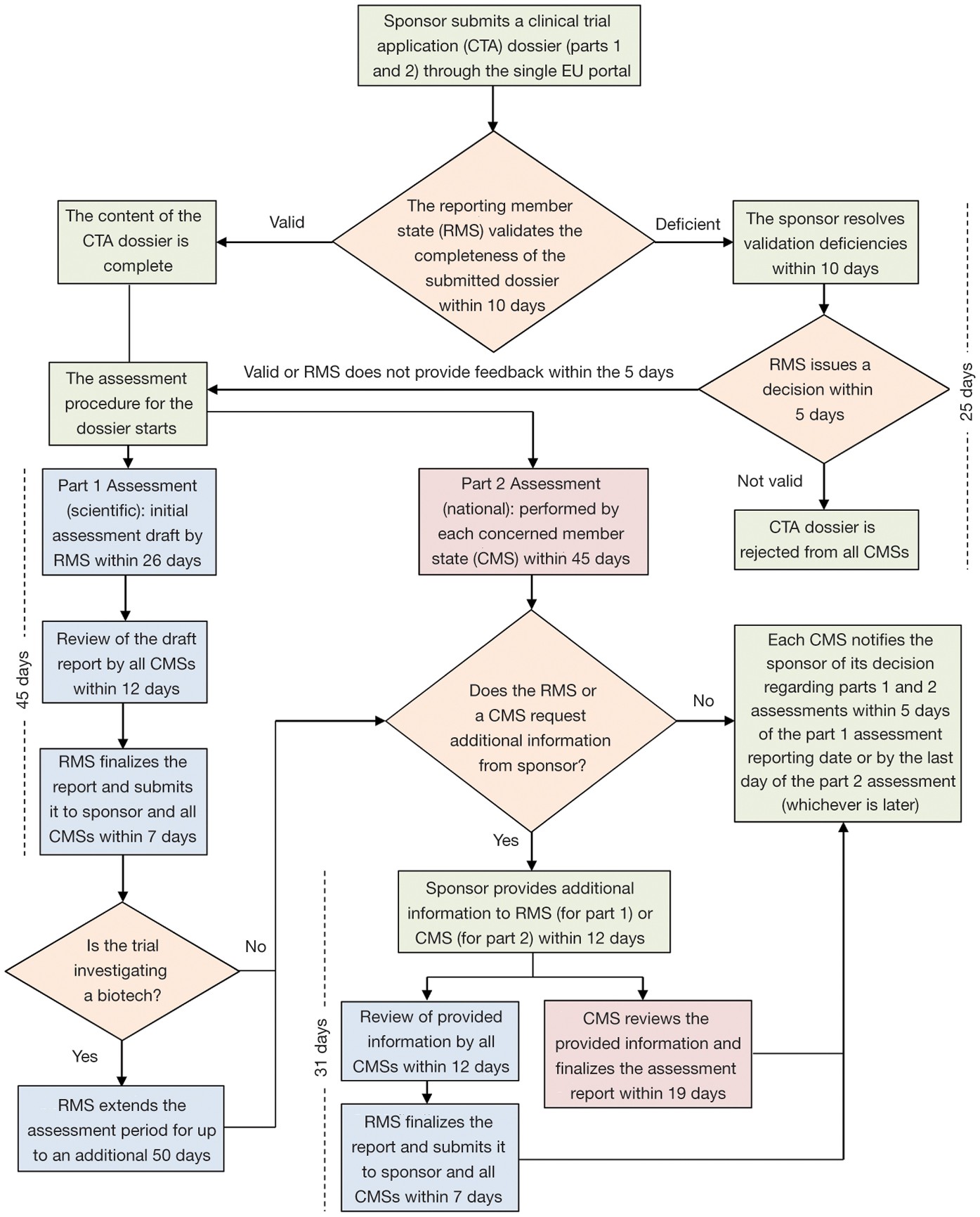
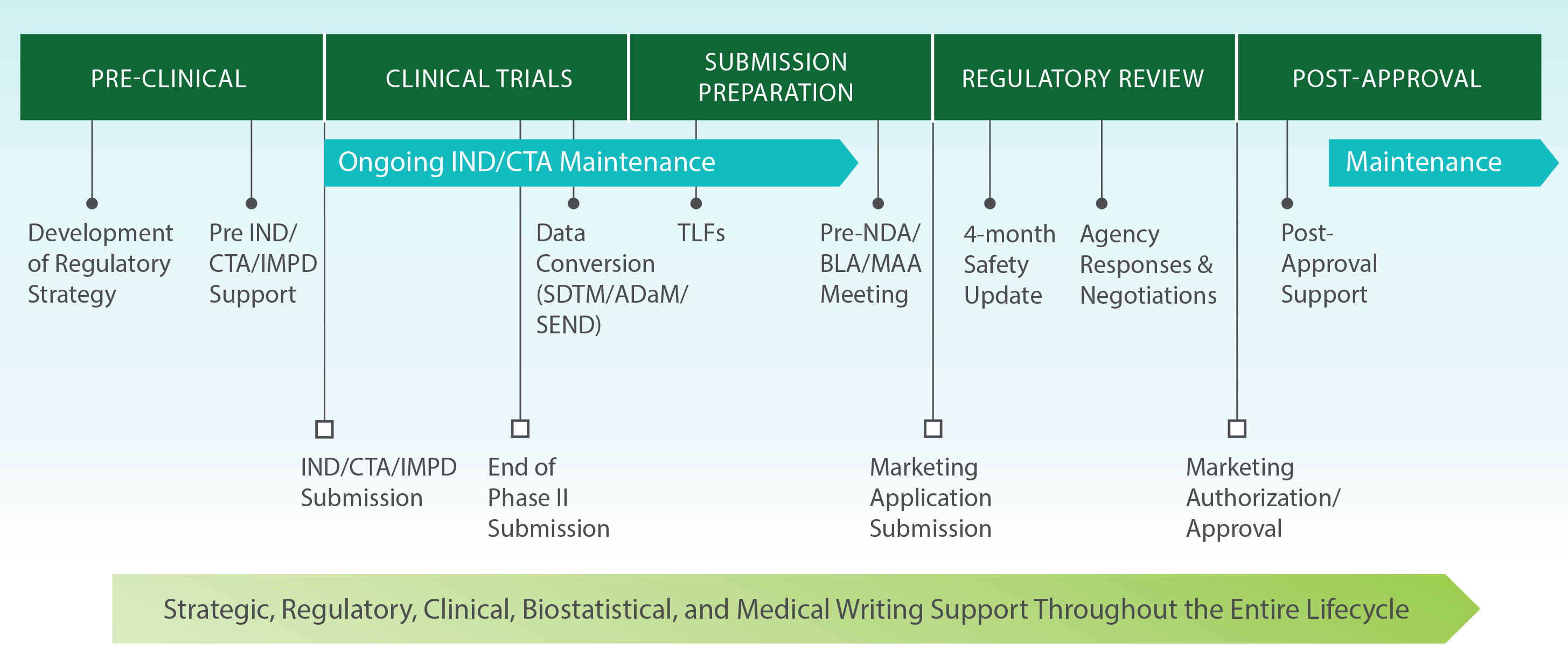
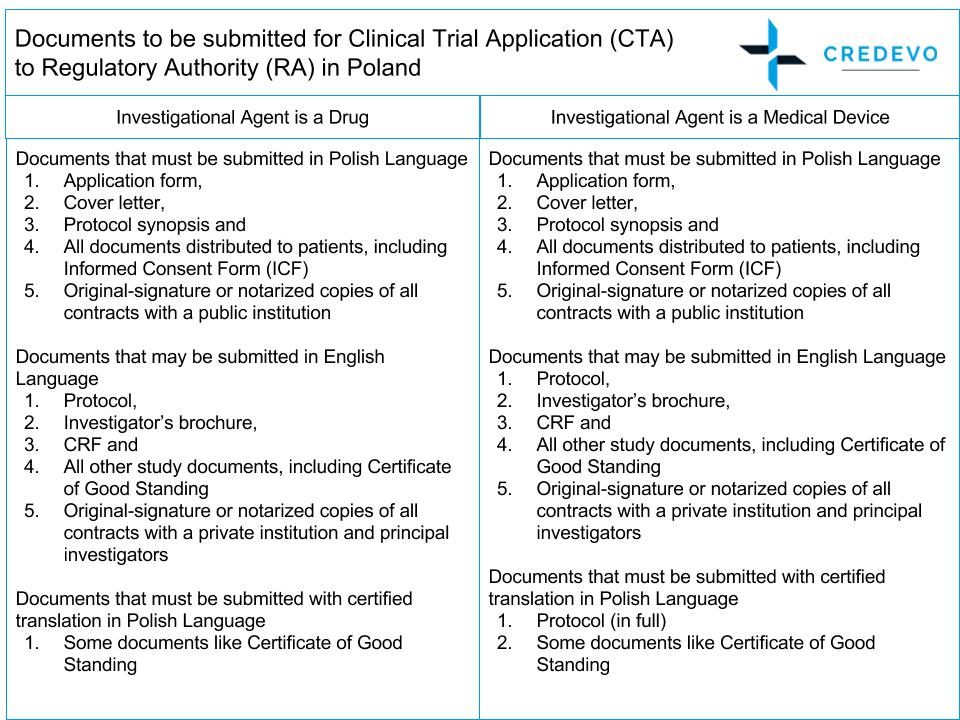
An overview of the procedure for clinical trial applications and the... | Download Scientific Diagram

Regulatory Affairs 101: Introduction to Investigational New Drug Applications and Clinical Trial Applications - Chiodin - 2019 - Clinical and Translational Science - Wiley Online Library