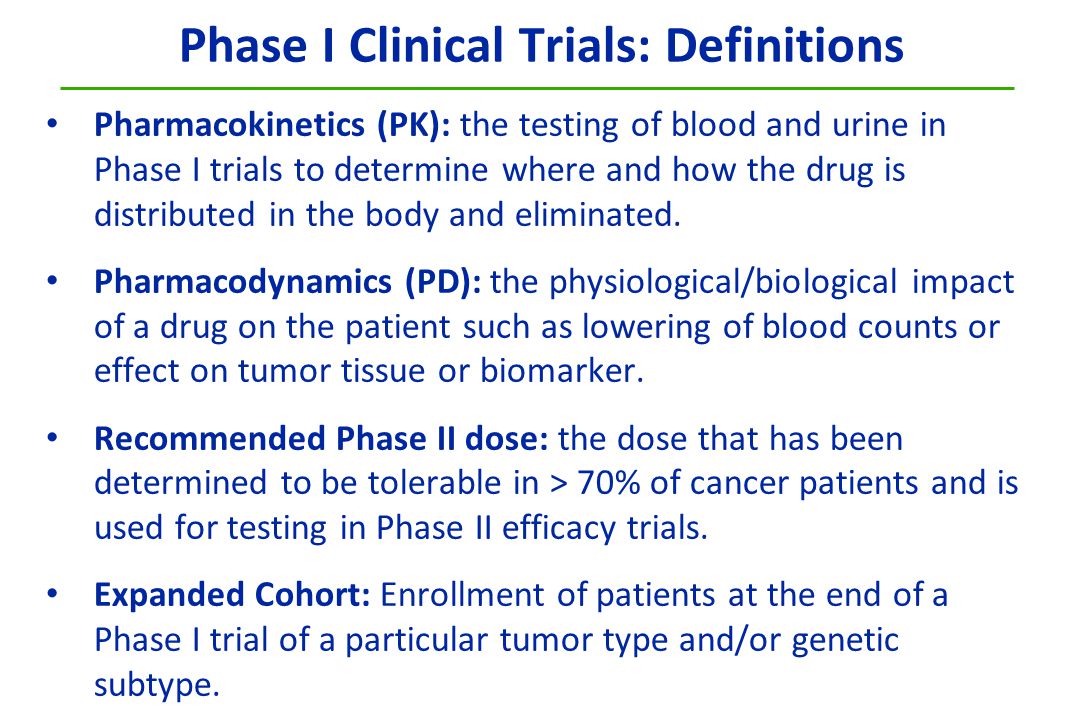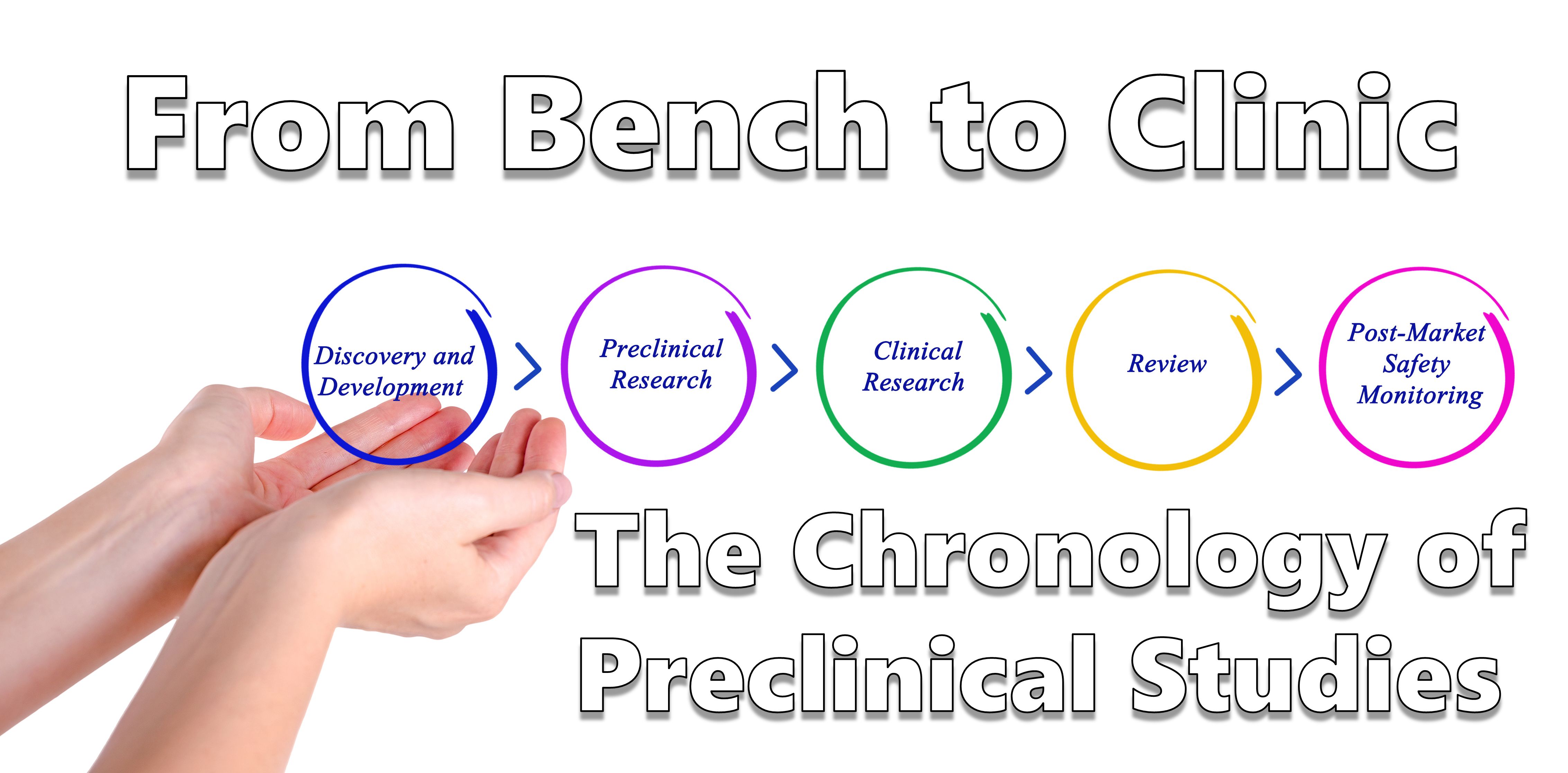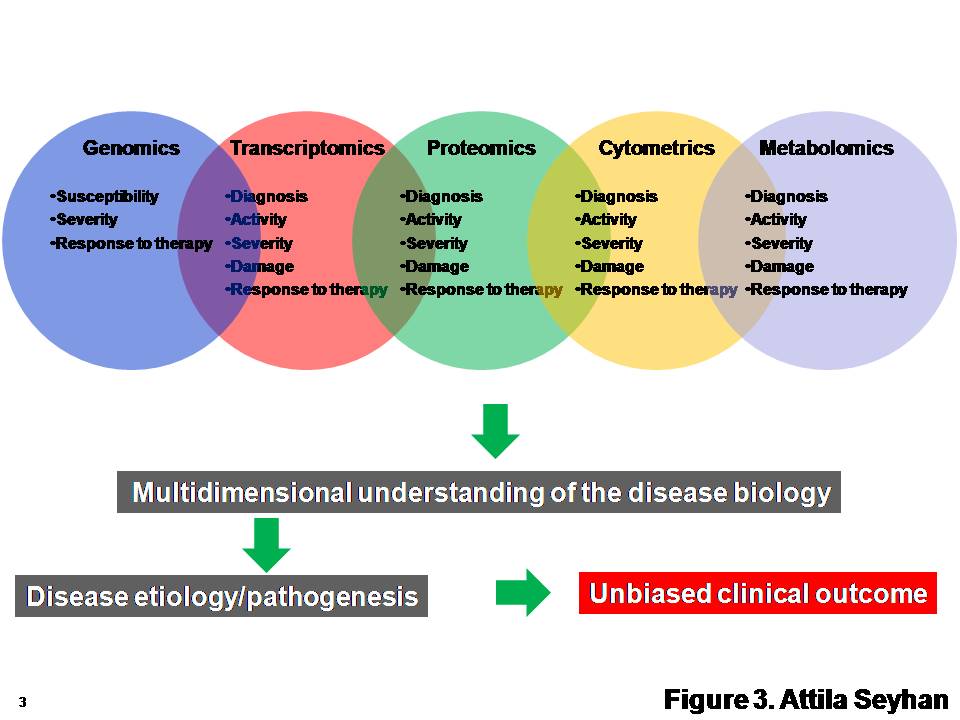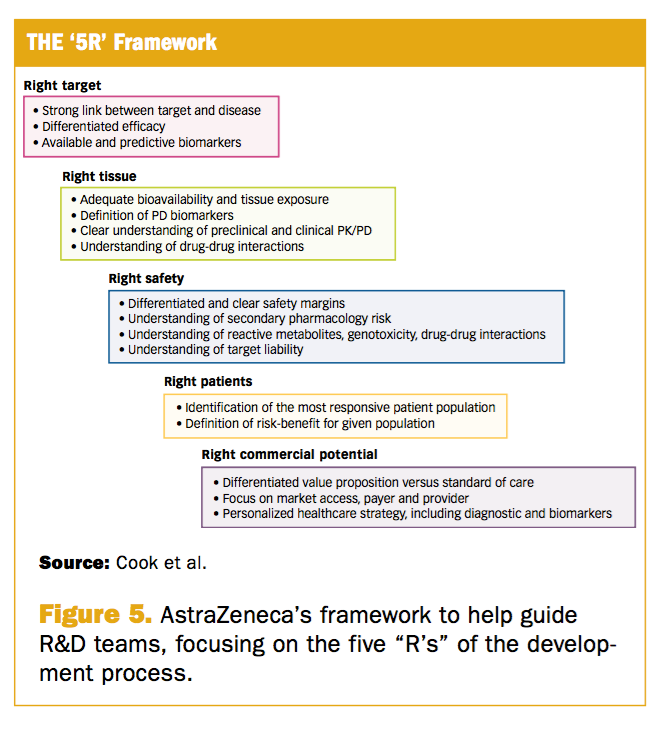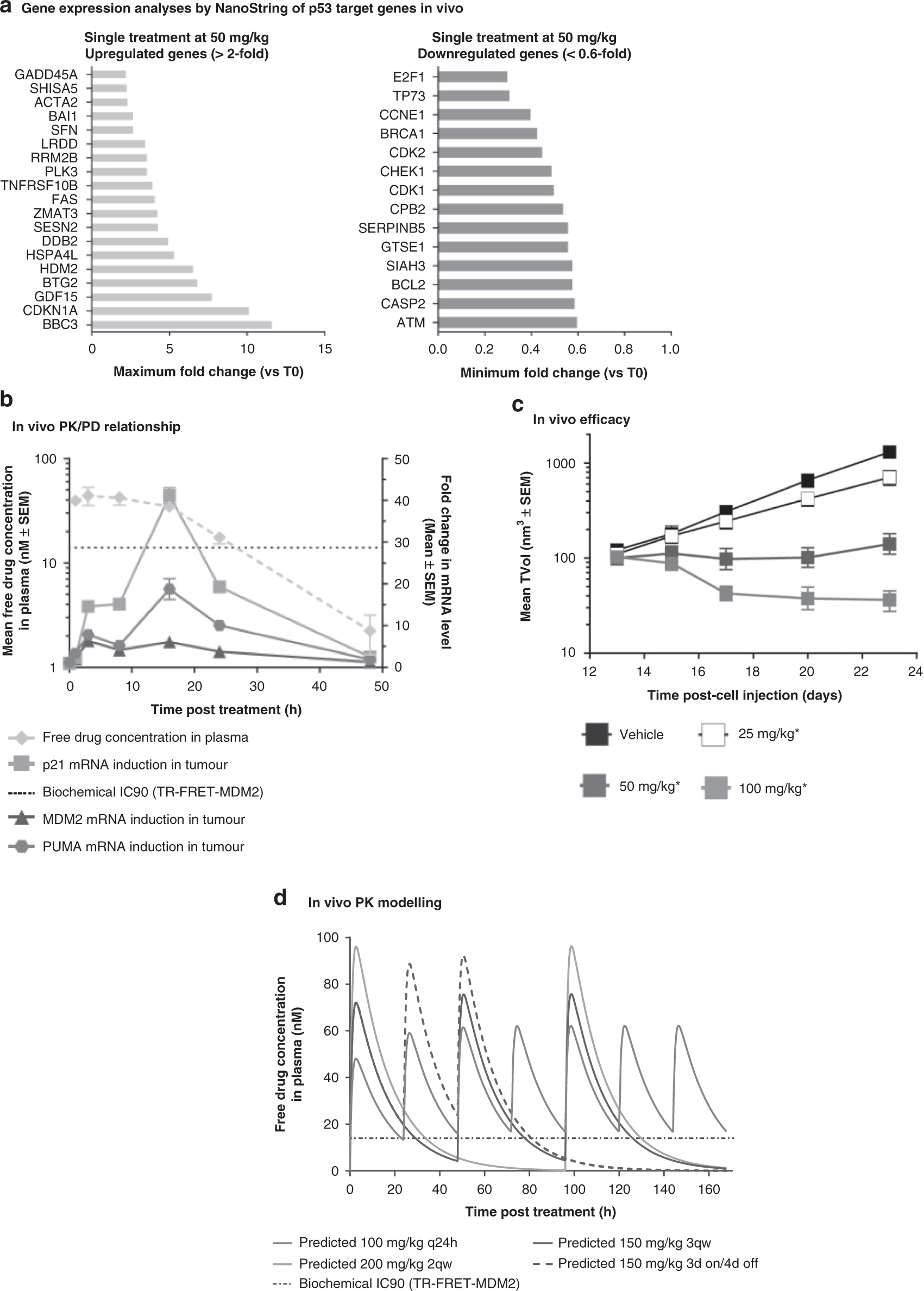
Pharmacokinetic–pharmacodynamic guided optimisation of dose and schedule of CGM097, an HDM2 inhibitor, in preclinical and clinical studies | British Journal of Cancer

The pharmacological audit trail (PhAT). (A) The six crucial aspects of... | Download Scientific Diagram
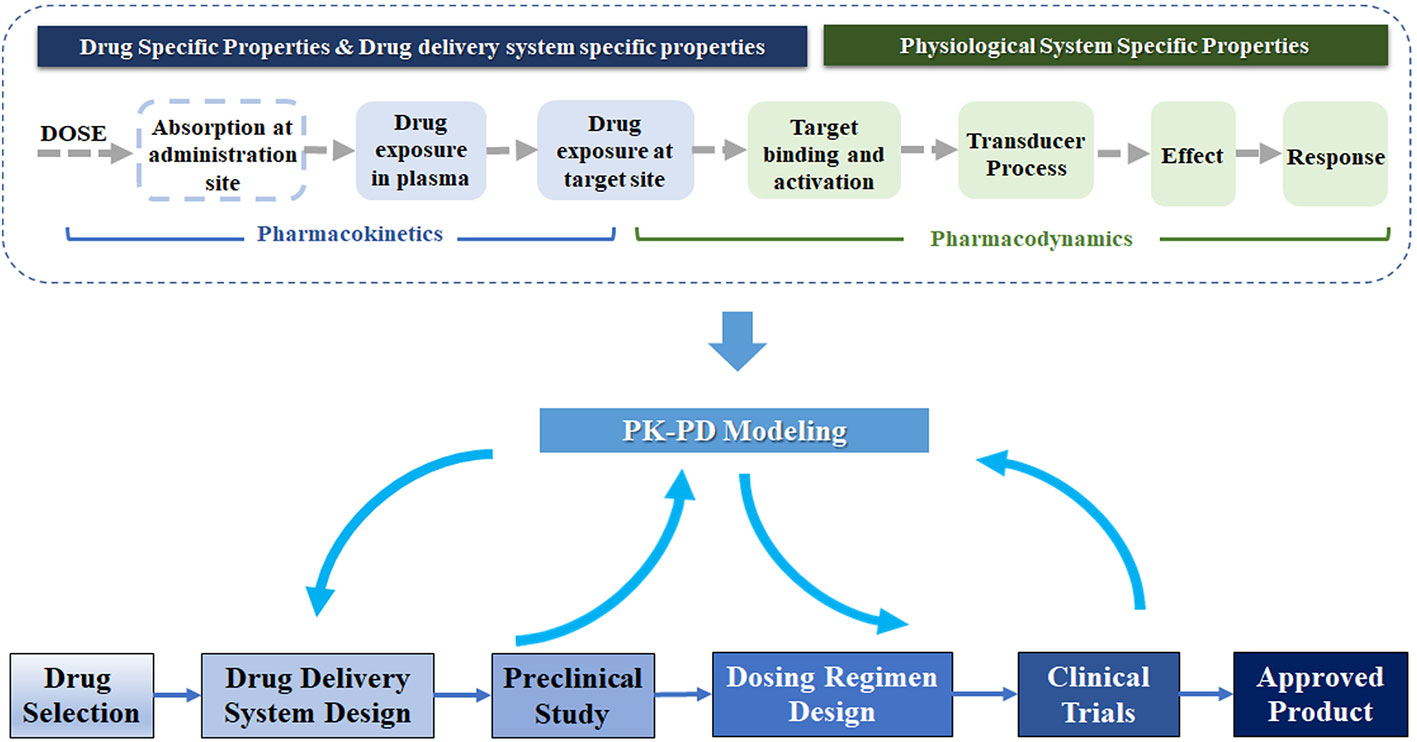
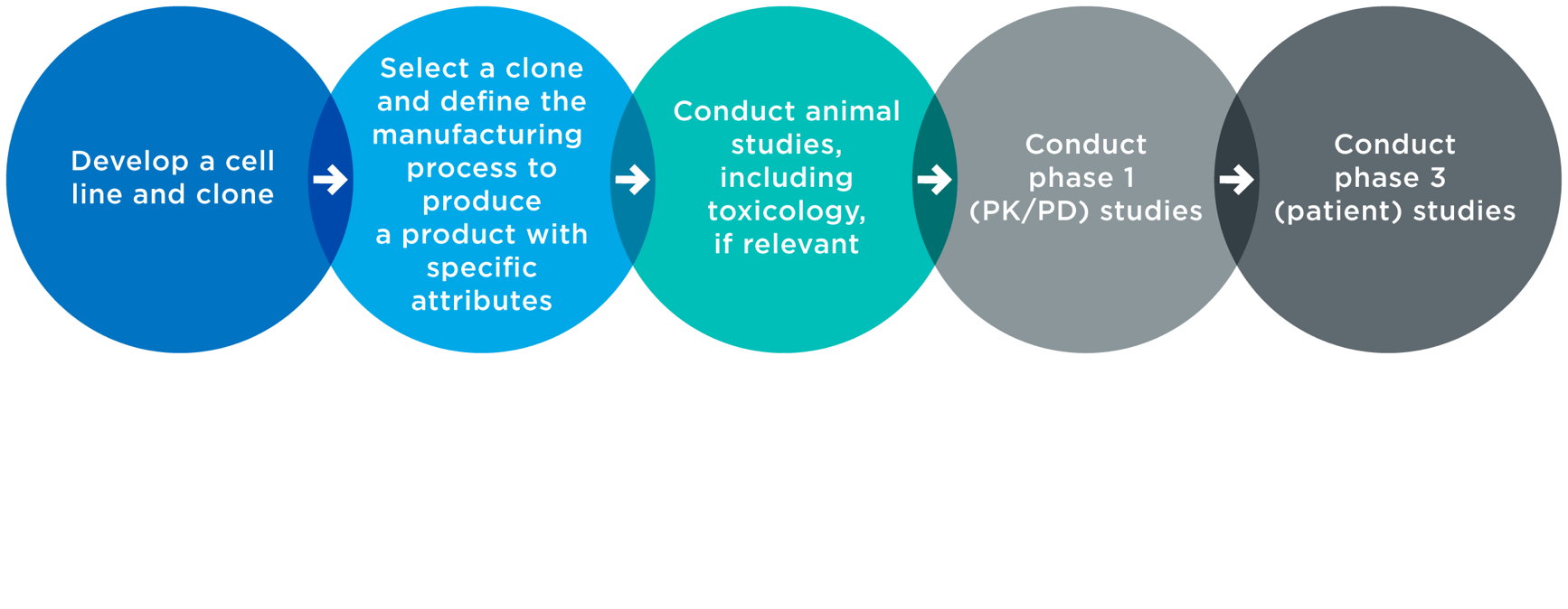
Frontiers | Application of Pharmacokinetic-Pharmacodynamic Modeling in Drug Delivery: Development and Challenges | Pharmacology

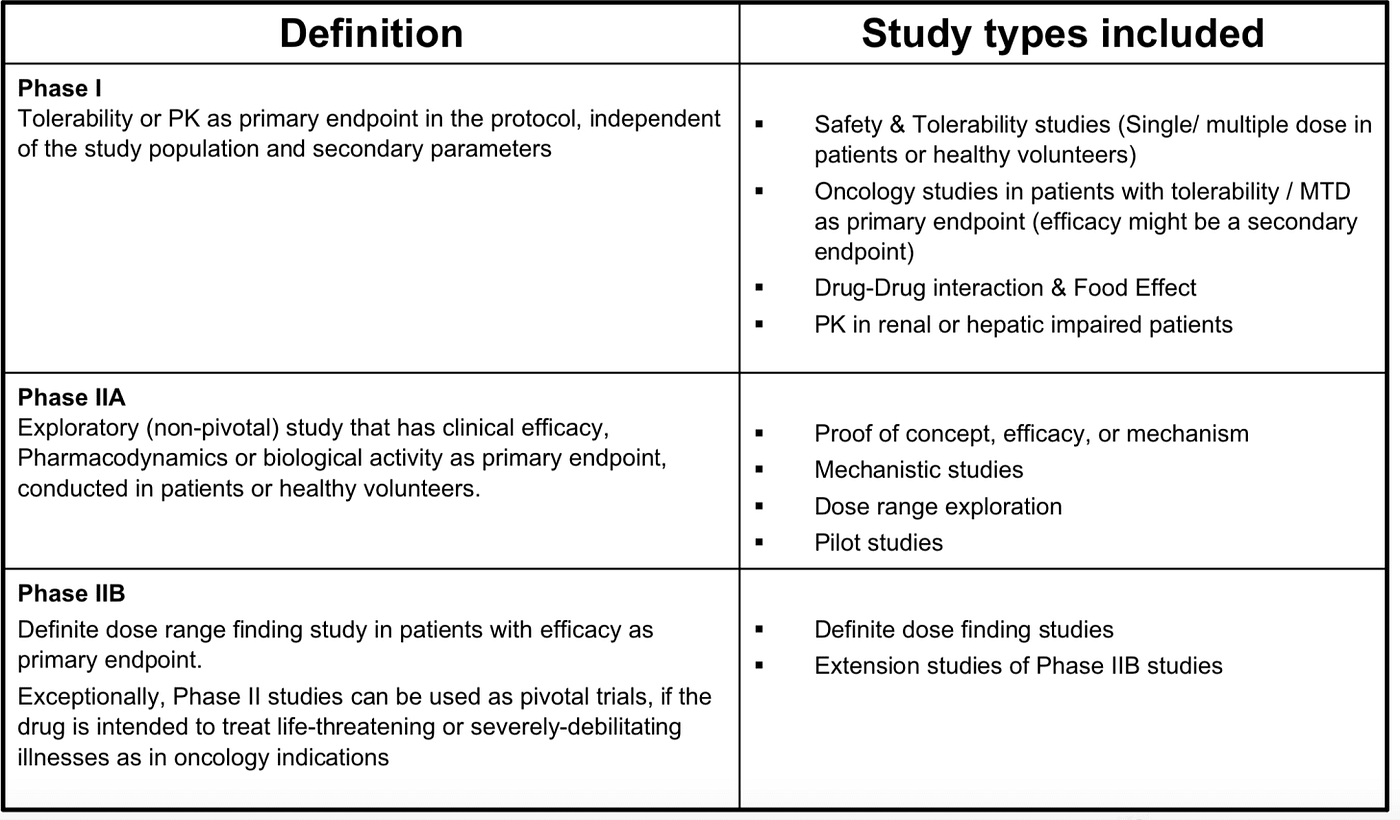
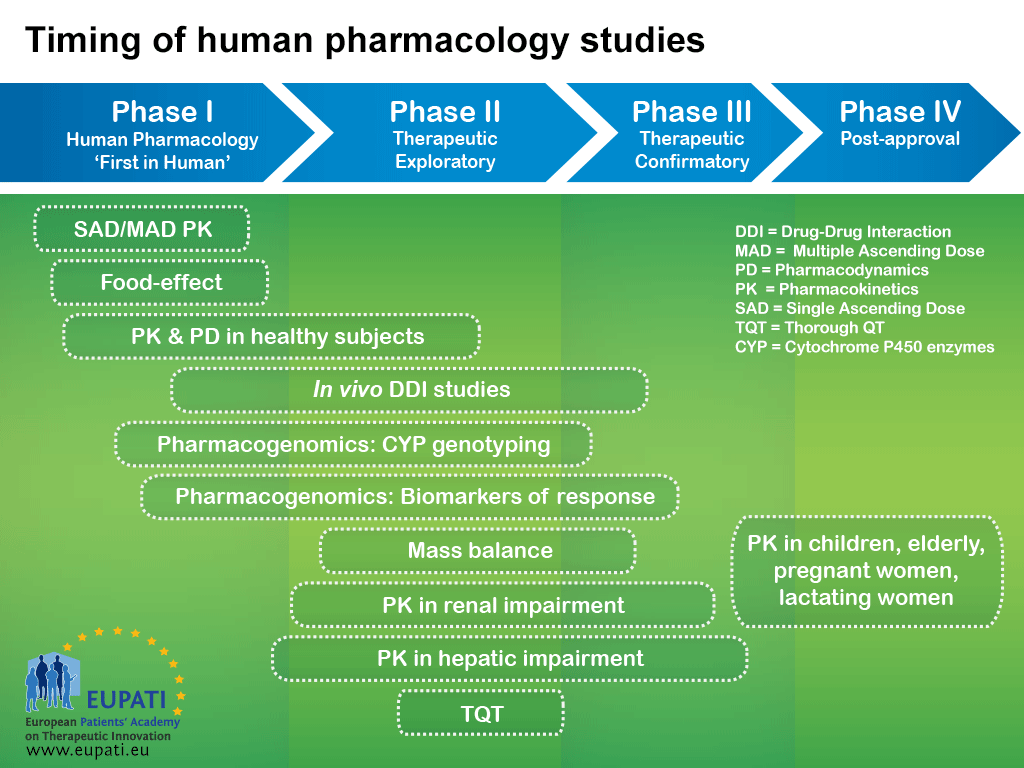
Pharmacokinetic and Statistical Considerations in First-in-Human Clinical Trials | Pharmaceutical Outsourcing - The Journal of Pharmaceutical & Biopharmaceutical Contract Services
![PDF] Critical parameters in targeted drug development: the pharmacological audit trail. | Semantic Scholar PDF] Critical parameters in targeted drug development: the pharmacological audit trail. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/0097f91095f75db9b8fcca3b6e4470d486036e5b/3-Figure1-1.png)
PDF] Critical parameters in targeted drug development: the pharmacological audit trail. | Semantic Scholar